New methodology for the N-alkylation of 2-amino-3-acylthiophenes†
Abstract
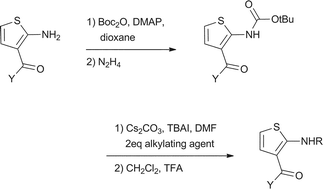
2-Amino-3-acylthiophenes are known to allosterically modulate the A1adenosine receptor and are also used as intermediates in the synthesis of therapeutic agents and pharmacophores such as thienoazepines and thienopyrimidines. The

 Please wait while we load your content...
Please wait while we load your content...