Highly efficient asymmetric vinylogous Mannich reaction induced by O-pivaloylated d-galactosylamine as the chiral auxiliary†
Abstract
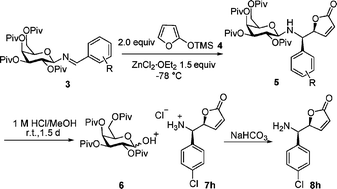
The diastereospecific formation of β-N-glycoside-linked α-amino-2(5H)-furanone has been achieved with high yield via a vinylogous Mannich reaction. The reaction was performed by using O-pivaloylated galactosylamine 1 as a chiral template and ZnCl2·Et2O as a promoter in Et2O.

 Please wait while we load your content...
Please wait while we load your content...