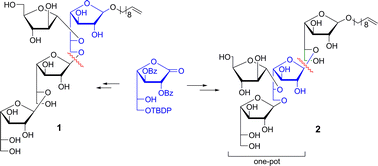
Mycolyl-arabinogalactan (mAG) complex is a major component of the cell wall of Mycobacterium tuberculosis, the causative agent of tuberculosis disease. Due to the essentiality of the cell wall for mycobacterium viability, knowledge of the biosynthesis of the arabinogalactan is crucial for the development of new therapeutic agents. In this context, we have synthesized two new branched arabinogalactafuranose tetrasaccharides, decenyl β-D-Galf-(1→5)-β-D-Galf-(1→6)[α-D-Araf(1→5)]-β-D-Galf (1) and decenyl β-D-Galf-(1→6)-[α-D-Araf-(1→5)]-β-D-Galf-(1→5)-β-D-Galf (2), as interesting tools for arabinofuranosyl transferase studies. The aldonolactone strategy for the introduction of the internal D-Galf was employed, allowing the construction of oligosaccharides from the non-reducing to the reducing end. Moreover, a one-pot procedure was developed for the synthesis of trisaccharide lactone 21, precursor of 2, which involved a glycosylation-deprotection-glycosylation sequence, through the use of TMSOTf as catalyst of the trichloroacetimidate method as well as promoter of TBDMS deprotection.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...