N-Heterocyclic carbene-catalyzed (NHC) three-component domino reactions: highly stereoselective synthesis of functionalized acyclic ε-ketoesters†
Abstract
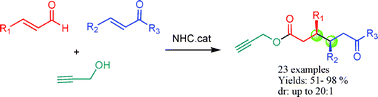
A novel NHC-catalyzed three-component domino strategy to access high functionalized cis-ε-ketoesters with excellent yields (up to 98%) and high stereoselectivities (up to 20 : 1) is documented. The title domino reactions are atom economical and work on a broad range of substrates. The relative stereochemistry could be explained by a cascade crossed-

 Please wait while we load your content...
Please wait while we load your content...