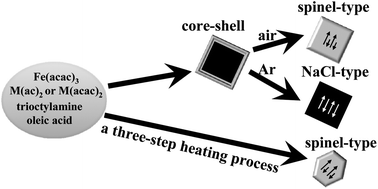
Significant studies have achieved beautiful control in particle size, while the shape- and phase-control synthesis of nanoparticles remains an open challenge. In this study, we have developed a generalized methodology to selectively prepare either NaCl-type (reduced form) or spinel-type ferrite (oxidized form) M–Fe–O (M = Mn, Co) crystallites with high reproducibility. A two-step heating process was able to control formation of two types of crystal phase, either a thermodynamic spinel-type under air or a kinetic-control of NaCl-type (rock salt structure) under Ar in a cubic morphology. On the other hand, the three-step heating procedure in air obtained the spinel-type with a thermodynamic equilibrium octahedral shape exclusively. Either using metal acetates (M(ac)2) or metal acetylacetonates (M(acac)2) as the starting precursors (M = Mn, Co) can be introduced to prepare NaCl-type (reduced form) or spinel-type ferrite (oxidized form) crystallites with identical experimental parameters, including precursor concentration, reaction temperature, reaction time, and heating rate. The oleic acid molecule, reaction temperature, and heating rate employed in the synthesis were carefully examined and found acting as determined roles behind the reaction processes. Apart from the previous literature reports as shape-directed and/or stabilizing agents, the oleic acid molecule played an additional phase-tuning role.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...