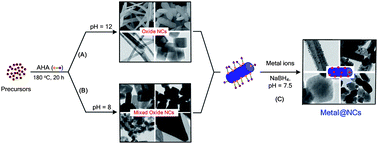
A conventional and general route has been exploited to the high yield synthesis of many kinds of highly crystalline metal oxide and mixed oxide nanocrystals with different morphologies including belt, rod, truncated-octahedron, cubic, sphere, sheet via the hydrothermal reaction of inorganic precursors in aqueous solution in the presence of bifunctional 6-aminohexanoic acid (AHA) molecules as a capping agent. This method is a simple, reproducible and general route for the preparation of a variety of high-crystalline inorganic nanocrystals in scale-up. The shape of inorganic nanocrystals such as CoWO4, La2(MoO4)3 can be controlled by simply adjusting the synthesis conditions including pH solution and reaction temperature. Further, by tuning precursor monomer concentration, the mesocrystal hierarchical aggregated microspheres (e.g., MnWO4, La2(MoO4)3) can be achieved, due to the spontaneous assembly of individual AHA-capped nanoparticles. These obtained AHA-capped nanocrystals are excellent supports for the synthesis of a variety of hybrid metal/oxide nanocrystals in which noble metal particles are uniformly deposited on the surface of each individual nanosupport. The photocatalytic activity of Ag/TiO2 nanobelts as a typical hybrid photocatalyst sample for Methylene Blue degradation was also studied.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...