Heterolytic cleavage of ammonia N–H bond by bifunctional activation in silica-grafted single site Ta(V)imido amido surface complex. Importance of the outer sphere NH3 assistance†
Abstract
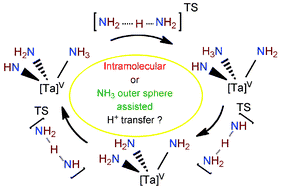
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)(–NH2)], 2, if excess
NH)(–NH2)], 2, if excess ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)(–NH2)(NH3)], 2q·NH3, show that the intramolecular H transfer from
NH)(–NH2)(NH3)], 2q·NH3, show that the intramolecular H transfer from ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH is ruled out, but the H transfers from the coordinated
NH is ruled out, but the H transfers from the coordinated

 Please wait while we load your content...
Please wait while we load your content...