A green protocol for the synthesis of conformationally rigid sulfur linked bisquinolines by double Friedlander reaction in water†
Abstract
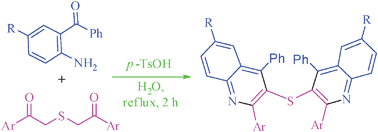
A series of bis(2-aryl-4-arylquinolin-3-yl)sulfanes and 1,2-bis(2-aryl-4-arylquinolin-3-yl)disulfanes were synthesised in good to excellent yields by double Friedlander reaction between 2-[(2-oxo-2-arylethyl)sulfanyl]-1-aryl-1-ethanones/2-[(2-oxo-2-arylethyl)disulfanyl]-1-aryl-1-ethanones and

 Please wait while we load your content...
Please wait while we load your content...