Electronic structure and metal–metal communication in (CpM)2(as-indacene) and (CpM)2(s-indacene) (M = Mn, Fe, Co, Ni) complexes: a DFT investigation†
Abstract
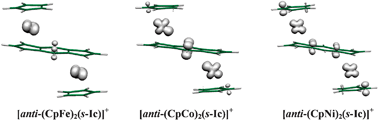
DFT calculations with full geometry optimization have been performed on the series (CpM)2(as-indacene) and (CpM)2(s-indacene) (M = Mn, Fe, Co, Ni), as well as on the
- This article is part of the themed collection: In honour of Didier Astruc

 Please wait while we load your content...
Please wait while we load your content...