A three-dimensional copper(ii) 12-metallacrown-4 complex with malonomonohydroxamic acid (H3mmh) as a ligand†
Abstract
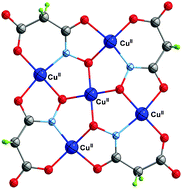
Slow diffusion of an ethanolic solution of copper(II) into an aqueous solution of partially deprotonated malonodihydroxamic acid (H4mdh) affords the pentanuclear complex of formula {[K(H2O)2]2[Cu5(mmh)4]}n (1) (H3mmh = malonomonohydroxamic acid) which is formed by 12-MC-4 metallacrown units with the fifth copper(II) ion being placed at the center of the square metallacrown unit. The dianionic pentacopper(II) planar entity interacts with diaquapotassium(I) counterions through the carboxylate-oxygen atoms resulting in a neutral three-dimensional structure. Magnetic susceptibility measurements in the temperature range 1.9–300 K for 1 show the occurrence of relatively strong antiferromagnetic interactions within the pentanuclear copper(II) core leading to two degenerated low-lying spin doublets at T < 50 K.

 Please wait while we load your content...
Please wait while we load your content...