A chemical model of intermediate states implied in the switching properties of CoFe Prussian blue analogues: how a cell parameter lengthening can cause a crystal field parameter increase†‡
Abstract
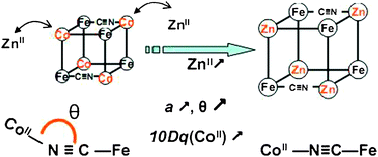
A series of CoFe Prussian blue analogues of chemical formula Rb2Co4−xZnx[Fe(CN)6]3.3·11H2O (x = 0, 1, 1.95 and 2.7) has been synthesized along which the MII/CoIII ions ratio at the Co site has been tuned. The long range order and the electronic structure of the Co ions have been investigated by combined
- This article is part of the themed collection: In honour of Didier Astruc

 Please wait while we load your content...
Please wait while we load your content...