Synthetic approaches to selenacephams and selenacephems via a cleavage of diselenide and selenium anion†
Abstract
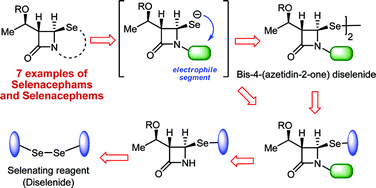
The bis-2-(trimethylsilyl)ethyl diselenide was prepared and used as a selenating reagent for the insertion of the 2-(trimethylsilyl)ethylseleno group at the C-4 position of the azetidinones. Bis-4-(azetidin-2-one)

 Please wait while we load your content...
Please wait while we load your content...