Hexanuclear Fe(iii) complexes and their thermal decomposition into an undecanuclear species†
Abstract
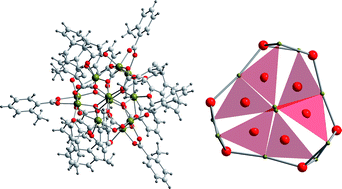
The anion of the amino-alcohol 3-dimethylamino-2-propanol has been used to prepare a family of hexanuclear Fe(III) complexes of general formula [Fe6O2(OH)2(RCO2)10(C5H12NO)2] (R = Me: 1, R = Ph: 2, R = m-MeC6H4: 3, R = o-MeC6H4: 4). The complexes have been characterized by

 Please wait while we load your content...
Please wait while we load your content...