On the multiple B–N bonding in boron compounds using the topological analysis of electron localization function (ELF)
Abstract
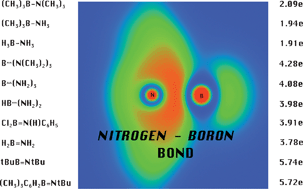
Topological analysis of the Electron Localization Function (ELF) within the framework of Quantum Chemical Topology (QCT) has been applied to study the nature of the boron–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N). According to the Lewis formula three types of bonds have been recognized. These are: the single B–N bond with a basin population of 1.91 ÷ 2.09e, the double B
N). According to the Lewis formula three types of bonds have been recognized. These are: the single B–N bond with a basin population of 1.91 ÷ 2.09e, the double B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond with a population of 3.78 ÷ 4.28e, and the triple B
N bond with a population of 3.78 ÷ 4.28e, and the triple B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond with a basin population of 5.72 ÷ 5.74e. In the case of partial double bonds (B
N bond with a basin population of 5.72 ÷ 5.74e. In the case of partial double bonds (B![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) N), where formally two or more resonance hybrids have to be considered, our calculations strongly support the concept of double boron–
N), where formally two or more resonance hybrids have to be considered, our calculations strongly support the concept of double boron–![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).

 Please wait while we load your content...
Please wait while we load your content...