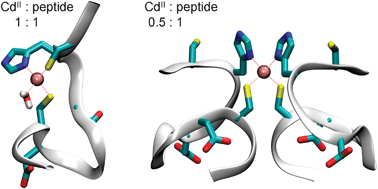
A de novo designed dodecapeptide (HS), inspired by the metal binding loops of metal-responsive transcriptional activators, was synthesized. The aim was to create a model system for structurally promiscuous and intrinsically unstructured proteins, and explore the effect of metal ions on their structure and dynamics. The interaction with CdII was investigated by UV, synchrotron radiation CD, 1H NMR, and perturbed angular correlation (PAC) of γ-rays spectroscopy, pH-potentiometry, and molecular modelling. The peptide mainly displays characteristics of random coil in the CD spectra, and the molecular dynamics simulations demonstrate that it is unstructured with transient and varying helical content. The spectroscopic studies revealed the formation of loop structures with the coordination of the two Cys-thiolates close to each end of the HSpeptide, in the presence of one equivalent of CdII per ligand. The imidazole moiety from histidine is also bound to CdII at neutral pH and above. In the presence of 0.5 equivalent of CdII per HS metal bridged structures with e.g.CdS2N2 and possibly CdS4 coordination geometries are formed above pH ∼6. In an equilibrium of several co-existing species the peptide is exchanging between a number of structures also in its metal ion bound state(s), as indicated by NMR and PAC data.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...