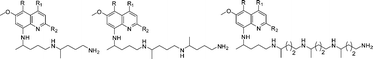
Extended side chain analogues of 8-aminoquinolines: Synthesis and evaluation of antiprotozoal, antimicrobial, β-hematin inhibition, and cytotoxic activities
Abstract
We report the synthesis of double, triple and quadruple extended side chain analogues of the
 Please wait while we load your content...
Please wait while we load your content...