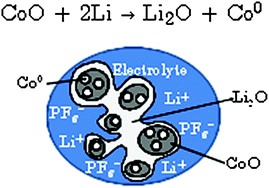
This work presents a new methodology based on a multi-interface superlattice approach to investigate interface electrochemistry in conversion reactions, through first-principles DFT or DFT + U calculations. This method is applied to the CoO electrode for which several experimental data are available, and describes the main interface effects by means of three interdependent descriptors, namely the chemical (interface bonding), the mechanical (stress) and the electrical/electrochemical (electric bias) descriptors. Thanks to this decomposition, the most probable interfaces occurring during the CoO conversion have been determined, and a probable electrode morphology has been proposed. Furthermore, the addition of an external redox potential to the multi-interface superlattice has revealed asymmetric electrochemical interface phenomena in charge and discharge, shedding some light into the voltage hysteresis observed for the CoO conversion/reconversion.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...