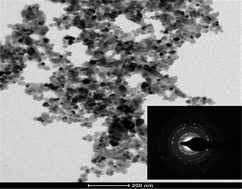
Herein, we have demonstrated a novel, facile, template free method for the synthesis of nanostructured zinc sulfide from bulk zinc oxide. This synthesis method may provide an easy, inexpensive and pollution free way to synthesize nanostructured zinc sulfide on a large scale. Zinc oxide and thiourea in various stoichiometric ratios were homogenized at moderate temperature to obtain zinc sulfide. The phase pure nanostructured cubic zinc sulfide was obtained using a 1 : 2 molar ratio of zinc oxide & thiourea and in excess of thiourea as well. Surprisingly, monodispersed nanotriangles of zinc sulfide were obtained using all molar ratios of zinc oxide and thiourea. The nanotriangles of size 5–6 nm were obtained for zinc sulfide of a 1 : 2 molar ratio. The band gap of nanostructured zinc sulfide was observed to be in the range of 3.86 to 3.75 eV, which is higher than that of the reported value. The blue shift was obtained due to the nanocrystalline nature of the zinc sulfide. Considering the significant morphology of zinc sulfide, it has been used as a photocatalyst for water splitting to produce hydrogen and for degradation of methylene blue as well. The hydrogen evolution rate was observed to be 2050 μmol h−1 g−1, which is much more than the bulk zinc sulfide (410 μmol h−1 g−1) as well as reported nanostructured ZnS. Three fold enhancements in the degradation of methylene blue as compared to bulk zinc sulfide have also been observed. The proposed simple methodology is believed to be a significant breakthrough in the field of nanotechnology and the method can be further generalized as a rational preparation scheme for the large-scale synthesis of various other nanostructured metal sulfides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...