Incorporation of phosphorus into the surface of natural graphite anode for lithium ion batteries†
Abstract
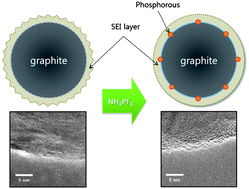
A new chemical approach for obtaining excellent cycleability of natural graphite is designed and introduced for use as an anode in lithium ion batteries. Here, we evaluate the electrochemical properties of natural graphite after surface modification with

 Please wait while we load your content...
Please wait while we load your content...