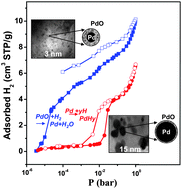
The hydrogen sorption/desorption mechanism below 1 bar at room temperature in porous carbons loaded with nanosized metal particles is not well understood and remains a controversial subject in the literature. The aim of this work is to provide a comprehensive view on the hydrogen sorption/desorption process on C/Pd composites by carefully analysing the phenomena involved during the hydrogen cycles in relation with the material characteristics (amount, size and chemical surface state of the palladium nanoparticles). The C/Pd composites consist of templated microporous carbon in which nanosized palladium particles were homogeneously dispersed. Depending on the synthesis condition, the amount of Pd loaded ranges between 1.4 and 12 wt% and the particle size is ranging from 3 to 15 nm. The presence of a palladium oxide layer on the Pd particle surface is revealed by XPS; the amount of this layer depends on the particle size. The hydrogen sorption/desorption measurements indicate that the total hydrogen amount sorbed on the C/Pd composite exceeds the amount required for the formation of β-palladium hydride. This hydrogen sorption excess is attributed in the literature to the spillover effect. To verify this assumption, we performed a careful exploitation of the hydrogen isotherms along with in situ and ex situ characterizations on the C/Pdx composites and a palladium oxide powder. For the first sorption/desorption cycle, two hydrogen sorption steps were identified and the sorbed hydrogen volume in each step was quantified. The first step which is irreversible is assigned to the reduction of PdO leading to the formation of Pd and water. The second step corresponds to the formation of the palladium hydride (PdHy), a step which is influenced by the presence of water. The processes involved in these two steps are strongly dependent on the Pd particle size. The results presented here clearly demonstrate that the PdO reduction is the predominant phenomenon, explaining the hydrogen uptake excess measured in the first cycle. Although a spillover effect cannot be excluded, the experimental data indicate that its possible contribution would remain significantly much weaker than the contribution due to the PdO reduction. To our knowledge, this is the first time that the effect of the Pd oxide layer on the hydrogen sorption (at low pressure and room temperature) on C/Pdx composites has been experimentally proved and quantified.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...