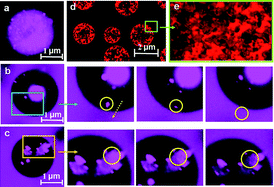
The overall goal of this research is to prepare amphiphilic poly(monomethoxypolyethylene glycol-co-D,L-lactide) (mPEG-PLA, PELA) microspheres with narrow size distribution for sustained release of recombinant human growth hormone (rhGH) without any exogenous stabilizing excipients. In order to obtain microspheres with narrow size distribution, we used a double emulsion method combined with a premix membrane emulsification technique. The morphology, internal structure, rhGH encapsulation efficiency, in vitro release and rhGH stability of PELA microspheres were characterized in detail. The results were compared with those of rhGH encapsulated in poly(D,L-lactic acid) (PLA) and poly(D,L-lactic-co-glycolic acid) (PLGA) microspheres. All microspheres possessed a narrow size distribution and had mean diameters of 2 μm. The encapsulation efficiency of PELA, PLA, and PLGA microspheres were 89.3%, 65.0% and 58.6%, respectively. PELA microspheres showed an initial burst release of 14.2% rhGH, followed by a constant release of 78.3% over a 45-day period. Whereas, the PLA and PLGA microspheres showed higher burst levels of 22.3% and 26.2%, followed by release of 49.1% and 60.5%, respectively. In addition, the PELA microspheres maintained much better integrity of rhGH than PLA and PLGA microspheres. These results suggested that PELA is an excellent polymer for encapsulating rhGH. We propose that the mPEG-PLA block copolymer acts in a similar way to a surfactant and that it may orientate itself on the biphasic interface, thereby minimizing the contact of protein with the oil/water interface for protein stabilization. Furthermore, the microcosmic mechanisms responsible for rhGH encapsulation and rhGH stability were elucidated using a confocal laser scanning microscope. These results are promising for the construction of a sustained release system for rhGH using PELA microspheres.

 Please wait while we load your content...
Please wait while we load your content...