Reversible assembly of metal nanoparticles induced by penicillamine. Dynamic formation of SERS hot spots†
Abstract
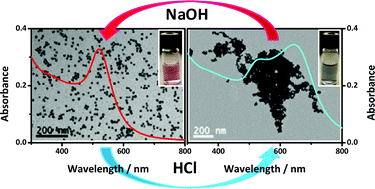
We report a systematic study of the surface modification of gold and
- This article is part of the themed collection: Self-organisation of nanoparticles

 Please wait while we load your content...
Please wait while we load your content...