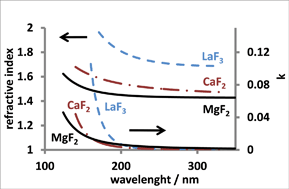
In this paper, we report on the atomic layer deposition of magnesium, calcium and lanthanum fluorides utilising two different approaches with hexafluoroacetylacetonate as a fluorine source. The first approach is based on the reaction of fluorinated metal precursors with a strong oxygen source. A deposition rate of 0.3 Å per cycle was obtained when calcium hexafluoroacetylacetonate (Ca(hfac)2) was used as a metal precursor and ozone as a second reactant at 300 °C. From Rutherford backscattering spectroscopy (RBS) measurements, the film stoichiometry was determined to be CaF2.17 with less than 5 atomic% of oxygen. The second and more feasible approach is based on metal oxide formation from traditional nonfluorinated β-diketonate type metal precursors and an oxygen source, followed by fluorination using hexafluoroacetylacetonate (Hhfac) and ozone. The MgF2, CaF2 and LaF3 thin films prepared using this method showed refractive indices of 1.429, 1.472 and 1.687, respectively. The oxygen content of these films was below the detection limit of the RBS, which is 2 at%.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...