Modification of activated carbons based on diazonium ionsin situ produced from aminobenzeneorganic acid without addition of other acid†
Abstract
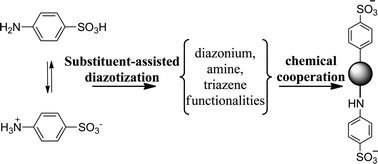
Activated carbon products modified with a benzene sulfonic acid group were prepared based on the spontaneous reduction of diazonium salts in situ generated in

 Please wait while we load your content...
Please wait while we load your content...