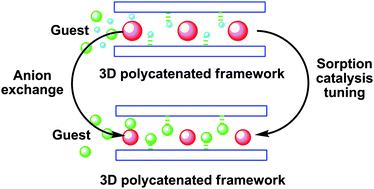
A structurally flexible porous metal–organic framework, [CuII(bped)2(H2O)2(SiF6)] ⊃ 4H2O (Cu-MOF-SiF6) was prepared from CuSiF6 and meso-1,2-bis(4-pyridyl)-1,2-ethanediol (bped) in EtOH–H2O. Cu-MOF-SiF6 is a three-dimensional (3D) polycatenated framework consisting of two-dimensional (2D) (4,4) topological grid networks which display 2-fold inclined interpenetration in the crystal lattice. The SiF62− anions hosted in the one-dimensional channels of Cu-MOF-SiF6 can be readily exchanged with NO3− anions while keeping the framework intact, leading to isomorphous Cu-MOF-NO3. The Cu-MOFs show selective adsorption behaviors due to the host–guest interaction, which is dependent on the anions and the guest size. The MeOH uptake investigation indicates that Cu-MOF-SiF6 demonstrates a breathing effect, while Cu-MOF-NO3 exhibits distinctive stepwise MeOH sorption due to the smaller size of NO3− and the larger voids in the crystal lattice. Cu-MOF-NO3 also exhibits stepwise sorption for larger EtOH guests, while the uptake of EtOH is blocked in Cu-MOF-SiF6. In addition, the solids show anion-responsive catalytic properties. Both Cu-MOF-SiF6 and Cu-MOF-NO3 efficiently oxidize benzylic compounds to the corresponding carbonyl functionality under mild and convenient reaction conditions, but improved catalytic activity was observed for Cu-MOF-NO3. The catalysts can be reused with the framework left intact at least three times without losing of any activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...