Electrochemical behavior and passivation of current collectors in lithium-ion batteries
Abstract
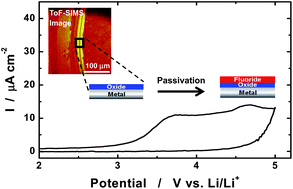
This paper examines several metals that are commonly employed as current collectors of positive and negative electrodes for rechargeable lithium batteries. Current collectors must be electrochemically stable when in contact with the cell component during the potential operation window of an electrode. Various electrochemical techniques have been used to investigate the corrosion of current collector materials. In practice, continued corrosion of current collectors leads to a gradual increase in the internal resistance of cells, which causes the capacity to fade gradually. Corrosion of the current collector may induce a short-circuit, affecting its safety. Thus, the formation of thick and compact, protective passive film on the metal surface is highly important so as to ensure battery performance and safety. Depending on the salts and additives, different types of protective films are formed. The solubility of these surface layers in the electrolyte is a determining factor in the overall stability of the current collector. In this review, we introduce the electrochemical behavior and protective film formation processes of various metallic current collectors in representative electrolytes.
- This article is part of the themed collection: Advanced Materials for Lithium Batteries

 Please wait while we load your content...
Please wait while we load your content...