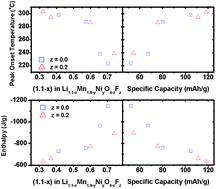
The thermal stability characteristics of spinel oxide and oxyfluoride cathodes Li1.1Mn1.9−yMyO4−zFz (M = Ni and Al, 0 ≤ y ≤ 0.3, and 0 ≤ z ≤ 0.2) have been investigated systematically. The oxides and the oxyfluoride samples have been synthesized by a low-temperature fluorination reaction at 450 °C of the spinel oxide precursors and have been characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), redox titration, electrochemical cycling, and differential scanning calorimetry (DSC). The thermal characteristics are assessed in terms of the onset temperature and reaction enthalpy for the exothermic reaction. The peak onset temperature increases and the reaction enthalpy decreases (i.e. thermal stability increases) with decreasing lithium content in the cathode in the charged state or increasing specific capacity (i.e. with increasing amount of lithium extraction).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...