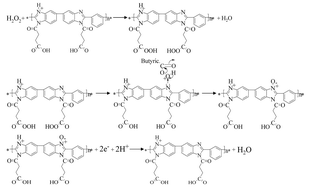
A novel polyelectrolyte, poly[N-(1-one-butyric acid)benzimidazole] (PBI-BA), was synthesized and used to fabricate an amperometric hydrogen peroxide (H2O2) sensor. It was found that PBI-BA can be oxidized by H2O2 and produce a current response during electrochemical reduction. This mechanism was used to develop an enzyme-free H2O2 sensor. Under optimal experimental conditions, the sensor showed high reproducibility, selectivity and stability. The stability of the PBI-BA electrode after annealing at 100 °C was also studied. Interestingly, the sensitivity of the modified electrode was 15.2% higher than that without heat treatment. The sensor was sensitive toward H2O2 over a linear range from 6.2 μM to 5.0 mM with a detection limit of 3.1 μM. Because graphene sheets (Gs) suspend readily in the polyelectrolyte solution, a PBI-BA–Gs/Au electrode was also fabricated, improving the sensor's performance dramatically: this sensor detected H2O2 over a linear range of 2.5 μM to 5 mM, with a correlation coefficient of 0.998 (n = 5) and a sensitivity of 1056 μA mM−1 cm−2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...