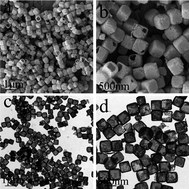
The synthesis of single-crystalline hollow particles with well-defined non-spherical shapes, especially hollow complex compounds, remains a significant challenge. In this paper, single-crystalline ZnSn(OH)6 (ZHS) hollow cubes were first synthesized by a facile self-templating method at room temperature. On the basis of X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy and transmission electron microscopy, it was found that hollow ZHS cubes were formed by a two-step process, in which solid cubes of ZHS were formed in the first step due to the co-precipitation of Zn(II) and Sn(IV) under basic conditions and then the solid cubes as the self-templates were converted to hollow ones through an alkali-assisted dissolution process. During the process, NaOH solution added in the second step is critical to the formation of ZHS hollow structures. The photocatalytic activity of ZHS hollow cubes for phenol degradation was tested, which showed much higher catalytic activity than that of the solid ZHS cubes. After four trials, the photocatalytic activity of the ZHS hollow cubes exhibits no significant loss.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...