Effect of transition metal (M = Co, Ni, Cu) substitution on electronic structure and vacancy formation of Li3N
Abstract
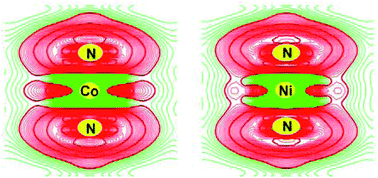
We carried out first principles calculations to investigate the effect of transition metal (M = Co, Ni, Cu) substitution on electronic structure and vacancy formation of Li3N in this study. Transition metals are shown to selectively substitute interplanar Li(1) atoms. Both Co and Ni substitution remarkably reduces the energy band gap to 0.55 eV in comparison with 1.13 eV of Li3N, while Cu substitution insignificantly decreases the energy band gap by 0.07 eV. Covalent bonding between transition metal atom and the coordinated N, which is manifested both visually by the contour plots of valence charge density difference and numerically by bond length variation, results in the formation of Li3−x−yMx□yN with y dependent on the covalency and concentration of transition metal. Ni substitution significantly reduces VLi(2) formation energy, which suggests greatly increased Li vacancy concentration for improved Li ionic mobility and conduction. Therefore, controlling the energy band gap and vacancy concentration by transition metal substitution provides a viable approach to tailor Li3N for variable applications in rechargeable lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...