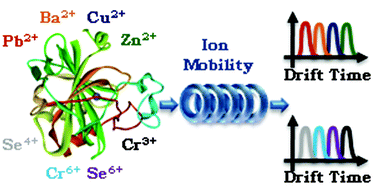
This work is proposed to demonstrate the Traveling-Wave Ion Mobility Specrometry (TWIMS) coupled to Mass Spectrometry (MS) as an alternative technique for speciation analysis between metals/metalloids and biomolecules. Mobilities of bovine carbonic anhydrase bound to Ba2+, Cu2+, Pb2+, Zn2+, Cr3+, Cr6+, Se4+ and Se6+ were estimated. The metal belonging to the bovine carbonic anhydrase structure, commonly found in the commercially available enzyme, was removed by filtration, using centrifugal filter devices. Then, some metals/metalloids were added to 10.0 mmol L−1 ammonium acetate at pH = 6.8 enzyme solution. Experiments were carried out by direct insertion of the sample at 10 μL min−1 flow rate into the ESI source of the instrument. Carbonic anhydrase mobility varied according to the metal bound in its structure, following the order: Zn2+ < Cu2+ < Ba2+ < Pb2+. Metals with higher affinity by the enzyme, such as Zn2+ and Cu2+ had lower mobility, suggesting a higher structural modification, binding itself to the enzyme metallic site. Considering metals with different oxidation states, the enzyme mobility followed the order: Se4+ < Cr6+ < Se6+ < Cr3+.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...