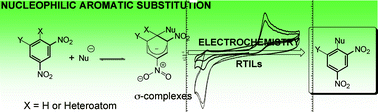
The current manuscript shows the electrochemical studies performed to rationalize the mechanism and develop new green synthetic routes for the synthesis of substituted nitroaromatics based on the advantages of the electrochemical approach to the nucleophilic aromatic substitution reaction (such as (a) low cost and ready availability of reagents, (b) atom economy, (c) high yields, approaching 100%) and the use of Room Temperature Ionic Liquids (RTILs) as green alternative solvents to organic aprotic solvents. Four of the most popular RTILs (1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4), 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM]PF6), 1-butyl-3-methyl-imidazolium bis(trifluoromethylsulfonyl)-imide ([BMIM]TFSI) and 1-butyl-3-methylimidazolium acetate ([BMIM]AcO) have been chosen since they have different properties in terms of solvation effects that can increase the regioselectivity of the reaction. The nucleophiles used to study the feasibility and viability of the reaction were the classical hydride, methoxide, ketones, cyanides and amines, whereas the nitroarenes selected were 4-nitrotoluene, 1,3-dinitrobenzene, 2,4-dinitroaniline, 1,3,5-trinitrobenzene, 1,3-dinitronaphthalene, 1-chloro-2,4,6-trinitrobenzene and 2,4,6-trinitroanisole. The electrocatalysis and regioselectivity effects of using RTILs are also investigated. The article concludes by analyzing the economic cost of performing this electrosynthesis in RTILs and organic solvent electrolyte systems, which contain 0.1 M of supporting electrolyte.

 Please wait while we load your content...
Please wait while we load your content...