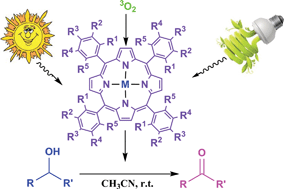
Highly selective, economical and efficient oxidation of alcohols to aldehydes and ketones by air and sunlight or visible light in the presence of porphyrins sensitizers
Abstract
In this work, a new aerobic route is introduced for the selective oxidation of a variety of aromatic and

 Please wait while we load your content...
Please wait while we load your content...