An unprecedented synthesis of γ-lactams via mercaptoacetylation of aziridines in water
Abstract
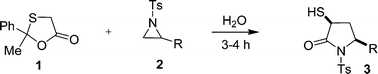
A highly green and expeditious route to α-mercapto-γ-lactams from masked mercapto acids viz. 2-phenyl-2-methyl-1,3-oxathiolan-5-ones, and tosyl aziridines in an excellent yield (82–93%) is reported. The synthetic protocol involves regioselective opening of the terminal aziridine ring and mercaptoacetylative

 Please wait while we load your content...
Please wait while we load your content...