High rate oxygen reduction in non-aqueous electrolytes with the addition of perfluorinated additives
Abstract
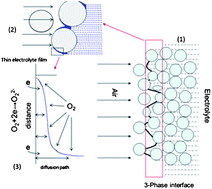
The discharge rate capability of Li–air batteries is substantially increased by using perfluorinated compounds as oxygen carriers. The solubility of oxygen in a non-aqueous electrolyte can be significantly increased by the introduction of such compounds, which leads to the increase in the diffusion-limited current of oxygen

 Please wait while we load your content...
Please wait while we load your content...