Hybrid dibismuthines and distibines as ligands towards transition metal carbonyls†
Abstract
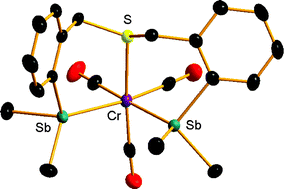
The hybrid dibismuthines O(CH2CH2BiPh2)2 and MeN(CH2-2-C6H4BiPh2)2 react with [M(CO)5(thf)] (M = Cr or W) to form [{M(CO)5}2{O(CH2CH2BiPh2)2}] and [{Cr(CO)5}2{MeN(CH2-2-C6H4BiPh2)2}] containing bridging bidentate (Bi2) coordination. The unsymmetrical tertiary bismuthine complexes [M(CO)5{BiPh2(o-C6H4OMe)}] are also described. Depending upon the molar ratio, the hybrid distibines O(CH2CH2SbMe2)2 and MeN(CH2-2-C6H4SbMe2)2 react with [M(CO)5(thf)] to give the pentacarbonyl complexes [{M(CO)5}2{O(CH2CH2SbMe2)2}] and [{Cr(CO)5}2{MeN(CH2-2-C6H4SbMe2)2}] or tetracarbonyls cis-[M(CO)4{O(CH2CH2SbMe2)2}] and cis-[M(CO)4{MeN(CH2-2-C6H4SbMe2)2}]. The latter can also be obtained from [Cr(CO)4(nbd)] or [W(CO)4(pip)2], and contain chelating bidentates (Sb2-coordinated) as determined crystallographically. S(CH2-2-C6H4SbMe2)2 coordinates as a tridentate (SSb2) in fac-[M(CO)3{S(CH2-2-C6H4SbMe2)2}] (M = Cr or Mo) and fac-[Mn(CO)3{S(CH2-2-C6H4SbMe2)2}][CF3SO3]. Fac-[Mn(CO)3{MeN(CH2-2-C6H4SbMe2)2}][CF3SO3] contains NSb2-coordinated

 Please wait while we load your content...
Please wait while we load your content...