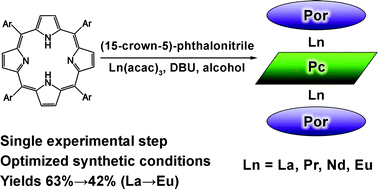
An effective one-step approach for the preparation of (porphyrinato)(phthalocyaninato) early lanthanides of type [Br4TPP]Ln[(15C5)4Pc]Ln[Br4TPP], where Br4TPP = 5,10,15,20-tetrakis-(4-bromophenyl)-porphyrinato-ligand, (15C5)4Pc = tetrakis-(15-crown-5)-phthalocyaninato-ligand and Ln = La, Pr, Nd or Eu, is developed. The influence of various factors on the reaction pathway and yields of the complexes is investigated in detail. The developed protocol is found to be general for the early lanthanide subgroup. Variation of the synthetic conditions allowed the determination and isolation of possible side-products, namely heteroleptic double-deckers [Br4TPP]Ln[(15C5)4Pc] (Ln = Nd, Eu) and triple-decker [Br4TPP]Nd[(15C5)4Pc]Nd[(15C5)4Pc]. The peculiarities of the NMR lanthanide-induced shifts (LIS) of resonances of the synthesized triple-decker complexes are precisely investigated. The isostructurality of the synthesized complexes within the series as well as isostructurality with previously synthesized compounds is demonstrated in terms of two-nuclei analysis of LIS.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...