Comparison of different ruthenium–alkylidene bonds in the activation step with N-heterocyclic carbene Ru-catalysts for olefins metathesis†
Abstract
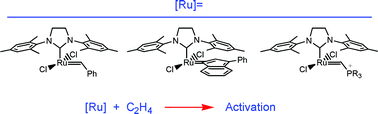
Density functional theory calculations have been used to investigate the activation steps involving three of the most used alkylidene groups in Ru-catalysts for
- This article is part of the themed collection: Computational chemistry of inorganic systems

 Please wait while we load your content...
Please wait while we load your content...