Anion-driven conformation control and enhanced sulfate binding utilising aryl linked salicylaldoxime dicopper helicates†
Abstract
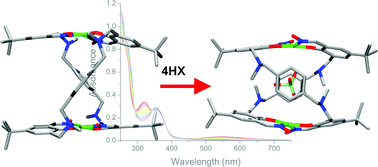
The synthesis and spectroscopic analysis of both “metal-only” and anion encapsulated dicopper(II) double helicates utilising a new 1,4-aryl spacer is described.
- This article is part of the themed collection: Self-assembly in inorganic chemistry

 Please wait while we load your content...
Please wait while we load your content...