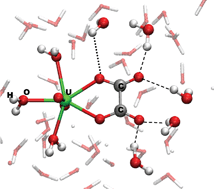
Car–Parrinello molecular dynamics simulations are reported for aqueous UO2(H2O)n(C2O4) (n = 3, 4), calling special attention to the binding modes of oxalate and the thermodynamics of the so-called chelate effect. Based on free energies from thermodynamic integration (BLYP functional), the κ1,κ1′-binding mode of the oxalate (with one O atom from each carboxylate coordinating) is more stable than κ2 (2 O atoms from the same carboxylate) and κ1 forms by 23 and 39 kJ mol−1, respectively. The free energy of binding a fourth water ligand to UO2(H2O)3(κ1-C2O4) is computed to be low, 12 kJ mol−1. Changes of the hydration shell about oxalate during chelate opening are discussed. Composite enthalpies and free energies, obtained from both experiment and quantum-chemical modeling, are proposed for the formation of monodentate UO2(H2O)4(κ1-C2O4). These data suggest that the largest entropy change in the overall complex formation occurs at this stage, and that the subsequent chelate closure under water release is essentially enthalpy-driven.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...