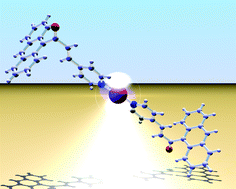
In solution, (E) to (Z)-isomerism is facile both in 3-(9-anthracenyl)-1-(pyridin-4-yl)propenone, 2, and in its silver(I) complex [Ag(2)2]+. The crystal structures of (E)-2, (Z)-2 and [Ag{(E)-2}2][SbF6] are presented, and the roles of edge-to-face and face-to-face π-interactions in the lattice are discussed. Solution NMR spectroscopic data suggest that the driving force for (E) to (Z) isomerization is intramolecular π-stacking of the pyridine and anthracene domains. The reversed enone 3-(9-anthracenyl)-1-(pyridin-4-yl)propen-3-one, (E)-3, and the silver(I) complex [Ag{(E)-3}2][SbF6] have been prepared and characterized, including a single crystal X-ray determination of the latter. Surprisingly, no π-stacking between anthracene or pyridine domains is observed in the solid state, and the crystal packing is dominated by Ag⋯F, CHanthracene⋯π-pyridine and CH⋯F interactions. In contrast to (E)-2 and [Ag{(E)-2}2]+, neither (E)-3 nor [Ag{(E)-3}2]+ undergoes photoisomerization in solution.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...