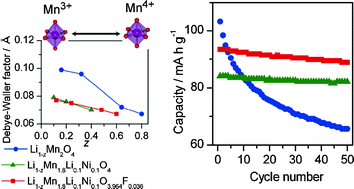
The electronic and local structures of partially anion-substituted lithium manganese spinel oxides as positive electrodes for lithium-ion batteries were investigated using X-ray absorption spectroscopy (XAS). LiMn1.8Li0.1Ni0.1O4−ηFη (η = 0, 0.018, 0.036, 0.055, 0.073, 0.110, 0.180) were synthesized by the reaction between LiMn1.8Li0.1Ni0.1O4 and NH4HF2. The shift of the absorption edge energy in the XANES spectra represented the valence change of Mn ion with the substitution of the low valent cation as Li+, Ni2+, or F− anion. The local structural change at each compound with the amount of a Jahn–Teller Mn3+ ion could be observed by EXAFS spectra. The discharge capacity of the tested electrode was in the order of LiMn2O4 > LiMn1.8Li0.1Ni0.1O4−ηFη (η = 0.036) > LiMn1.8Li0.1Ni0.1O4 while the cycleability was in the order of LiMn1.8Li0.1Ni0.1O4−ηFη (η = 0.036) ≈ LiMn1.8Li0.1Ni0.1O4 > LiMn2O4. It was clarified that LiMn1.8Li0.1Ni0.1O4−ηFη has a good cycleability because of the anion doping effect and simultaneously shows acceptable rechargeable capacity because of the large amount of the Jahn–Teller Mn3+ ions in the pristine material.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...