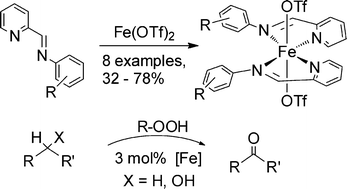
A set of iron(II) complexes of the general formula [Fe(OTf)2L2] was synthesized in 32 to 78% isolated yields, where L represents a bidentate α-iminopyridine ligand. Four of the iron complexes were characterized structurally, revealing a rich coordination chemistry, because the coordination geometry of the iron complexes strongly depends on the substitution pattern exhibited by the ligands L. The catalytic activity of the new complexes was demonstrated in the oxidation of cyclohexane, activated methylene groups and secondary alcohols to the corresponding ketones utilizing H2O2 and t-BuOOH as the oxidants. The oxidation of activated methylene groups and secondary alcohols to the corresponding ketones with t-BuOOH gave isolated yields between 22 and 91% (4 h, room temperature, 3% catalyst load). The influence of the structure of the ligand on the activity of the corresponding metal complex is also reported. Furthermore, UV-vis experiments were performed which provided evidence for the formation of an [Fe-O-O-t-Bu] intermediate.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...