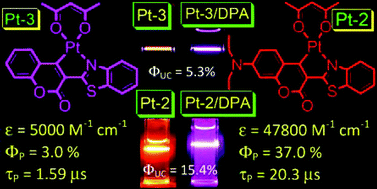
We studied four cyclometallated Pt(II) complexes, in which the thiazo-coumarin ligands (Pt-2, Pt-3 and Pt-4) or the phenylthiazo ligand (Pt-1) were directly cycloplatinated. Pt-2 shows intense absorption in visible region but other complexes show blue-shifted absorption. Room temperature phosphorescence was observed for all the complexes, and the emission wavelength is dependent on the size of the π-conjugation, not the intramolecular charge transfer (ICT) feature of the C⁁N ligands. Pt-2 shows longer phosphorescence lifetime (τ = 20.3 μs) than other complexes (below 2.0 μs). Furthermore, Pt-2 shows phosphorescence quantum yield Φ = 0.37, whereas Pt-3 and Pt-4 show much smaller Φ values (0.03 and 0.01, respectively). DFT/TDDFT calculations indicate 3IL triplet excited states for the complexes. The complexes were used as for luminescence O2 sensing and triplet–triplet-annihilation (TTA) based upconversion. Stern–Volmer quenching constant KSV = 0.026 Torr−1 was observed for Pt-2, ca. 89-fold of that of Pt-3. TTA upconversion is achieved with Pt-2 (λem = 400 nm with λex = 473 nm, anti-Stokes shift is 0.47 eV, excitation power density is at 70 mW cm−2). The upconversion quantum yield with Pt-2 as triplet sensitizer is up to 15.4%. The TTET efficiency (KSV = 1.33 × 105 M−1, kq = 6.57 × 109 M−1s−1. DPA as quencher) of Pt-2 is 34-fold of the model complex [Ru(dmb)3][PF6]2. Our results show that the 3IL state can be readily accessed by direct cyclometallation of organic fluorophores and this approach will be useful for preparation and applications of transition metal complexes that show intense absorption in visible region and the long-lived emissive 3IL excited states.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...