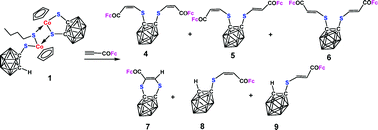
The reaction of the dinuclear cobalt compound [(CpCoS2C2B10H10)(CpCoSC2B10H11)(n-C4H9S)] (1) with HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(O)Fc leads to the cobalt-free products (C2B10H10)(SCH
CC(O)Fc leads to the cobalt-free products (C2B10H10)(SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOFc)2 (4–6), (S2C2B10H10)(HC
CHCOFc)2 (4–6), (S2C2B10H10)(HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCOFc) (7), and (C2B10H11)(SCH
CCOFc) (7), and (C2B10H11)(SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOFc) (8, 9). 4–6 are produced by hydrosulfuration of the alkyne at the 1,2-dicarba-closo-dodecaborane-dithiolate ligand with the generated vinyl groups in Z/Z, Z/E and E/E configurations, respectively. In 7, the alkyne is added to 1,2-dicarba-closo-dodecaborane-dithiolate at the two sulfur sites. 8 and 9 are the products of alkyne hydrosulfuration at the 1,2-dicarba-closo-dodecaborane-1-monothiolate ligand with the generated vinyl group in either Z or E configuration. The treatment of 1 with HC
CHCOFc) (8, 9). 4–6 are produced by hydrosulfuration of the alkyne at the 1,2-dicarba-closo-dodecaborane-dithiolate ligand with the generated vinyl groups in Z/Z, Z/E and E/E configurations, respectively. In 7, the alkyne is added to 1,2-dicarba-closo-dodecaborane-dithiolate at the two sulfur sites. 8 and 9 are the products of alkyne hydrosulfuration at the 1,2-dicarba-closo-dodecaborane-1-monothiolate ligand with the generated vinyl group in either Z or E configuration. The treatment of 1 with HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Me gives rise to the parallel products (C2B10H10)(SCH
CCO2Me gives rise to the parallel products (C2B10H10)(SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me)2 (10–12) and (C2B10H11)(SCH
CHCO2Me)2 (10–12) and (C2B10H11)(SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me) (13, 14). All of the new compounds have been characterized by IR, NMR, elemental analysis and mass spectroscopy. The structures of compounds 4, 7, and 8 have also been determined by single-crystal X-ray diffraction analysis.
CHCO2Me) (13, 14). All of the new compounds have been characterized by IR, NMR, elemental analysis and mass spectroscopy. The structures of compounds 4, 7, and 8 have also been determined by single-crystal X-ray diffraction analysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(O)Fc leads to the cobalt-free products (C2B10H10)(SCH
CC(O)Fc leads to the cobalt-free products (C2B10H10)(SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOFc)2 (4–6), (S2C2B10H10)(HC
CHCOFc)2 (4–6), (S2C2B10H10)(HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCOFc) (7), and (C2B10H11)(SCH
CCOFc) (7), and (C2B10H11)(SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOFc) (8, 9). 4–6 are produced by hydrosulfuration of the
CHCOFc) (8, 9). 4–6 are produced by hydrosulfuration of the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Me gives rise to the parallel products (C2B10H10)(SCH
CCO2Me gives rise to the parallel products (C2B10H10)(SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me)2 (10–12) and (C2B10H11)(SCH
CHCO2Me)2 (10–12) and (C2B10H11)(SCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me) (13, 14). All of the new compounds have been characterized by
CHCO2Me) (13, 14). All of the new compounds have been characterized by 

 Please wait while we load your content...
Please wait while we load your content...