Structure–activity relationships in group 3 metal catalysts for asymmetric intramolecular alkenehydroamination. An investigation of ligands based on the axially chiral 1,1′-binaphthyl-2,2′-diamine motif†
Abstract
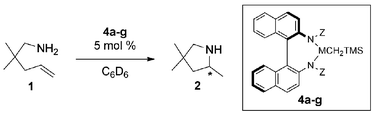
From a series of N,N′-disubstituted-1,1′-binaphthyl-2,2′-diamines, several group 3 metal complexes were synthesized via an in situ procedure. These chiral complexes were subsequently applied to catalysis of intramolecular
- This article is part of the themed collection: d0 organometallics in catalysis

 Please wait while we load your content...
Please wait while we load your content...