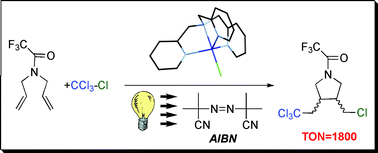
Photoinitiated ambient temperature copper-catalyzed atom transfer radical addition (ATRA) and cyclization (ATRC) reactions in the presence of free-radical diazo initiator (AIBN)
Abstract
The use of UV light in copper-catalyzed atom transfer radical addition (ATRA) and

 Please wait while we load your content...
Please wait while we load your content...