PtII-coordinated NCNR′2 species are so highly activated towards 1,3-dipolar cycloaddition (DCA) that they react smoothly with the acyclic nitrones ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+(O−)R′′ (Ar/R′′ = C6H4Me-p/Me; C6H4OMe-p/CH2Ph) in the Z-form. Competitive reactivity study of DCA between trans-[PtCl2(NCR)2] (R = Ph and NR′2) species and the acyclic nitrone 4-MeC6H4CH
N+(O−)R′′ (Ar/R′′ = C6H4Me-p/Me; C6H4OMe-p/CH2Ph) in the Z-form. Competitive reactivity study of DCA between trans-[PtCl2(NCR)2] (R = Ph and NR′2) species and the acyclic nitrone 4-MeC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+(O−)Me demonstrates comparable reactivity of the coordinated NCPh and NCNR′2, while alkylnitrile ligands do not react with the dipole. The reaction between trans-[PtCl2(NCNR′2)2] (R′2 = Me2, Et2, C5H10) and the nitrones proceed as consecutive two-step intermolecular cycloaddition to give mono-(1a–d) and bis-2,3-dihydro-1,2,4-oxadiazole (2a–d) complexes (Ar/R′′ = p-tol/Me: R′2 = Me2a, R′2 = Et2b, R′2 = C5H10c; Ar/R′′ = p-MeOC6H4/CH2Ph: R′2 = Me2d). All complexes were characterized by elemental analyses (C, H, N), high resolution ESI-MS, IR, 1H and 13C{1H} NMR spectroscopy. The structures of trans-1b, trans-2a, trans-2c, and trans-2d were determined by single-crystal X-ray diffraction. Metal-free 5-NR′2-2,3-dihydro-1,2,4-oxadiazoles 3a–3d were liberated from the corresponding (dihydrooxadiazole)2PtII complexes by treatment with excess NaCN and the heterocycles were characterized by high resolution ESI+-MS, 1H and 13C{1H} spectroscopy.
N+(O−)Me demonstrates comparable reactivity of the coordinated NCPh and NCNR′2, while alkylnitrile ligands do not react with the dipole. The reaction between trans-[PtCl2(NCNR′2)2] (R′2 = Me2, Et2, C5H10) and the nitrones proceed as consecutive two-step intermolecular cycloaddition to give mono-(1a–d) and bis-2,3-dihydro-1,2,4-oxadiazole (2a–d) complexes (Ar/R′′ = p-tol/Me: R′2 = Me2a, R′2 = Et2b, R′2 = C5H10c; Ar/R′′ = p-MeOC6H4/CH2Ph: R′2 = Me2d). All complexes were characterized by elemental analyses (C, H, N), high resolution ESI-MS, IR, 1H and 13C{1H} NMR spectroscopy. The structures of trans-1b, trans-2a, trans-2c, and trans-2d were determined by single-crystal X-ray diffraction. Metal-free 5-NR′2-2,3-dihydro-1,2,4-oxadiazoles 3a–3d were liberated from the corresponding (dihydrooxadiazole)2PtII complexes by treatment with excess NaCN and the heterocycles were characterized by high resolution ESI+-MS, 1H and 13C{1H} spectroscopy.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+(O−)R′′ (Ar/R′′ = C6H4Me-p/Me; C6H4OMe-p/CH2Ph) in the Z-form. Competitive reactivity study of DCA between trans-[PtCl2(NCR)2] (R = Ph and NR′2) species and the acyclic nitrone 4-MeC6H4CH
N+(O−)R′′ (Ar/R′′ = C6H4Me-p/Me; C6H4OMe-p/CH2Ph) in the Z-form. Competitive reactivity study of DCA between trans-[PtCl2(NCR)2] (R = Ph and NR′2) species and the acyclic nitrone 4-MeC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+(O−)Me demonstrates comparable reactivity of the coordinated NCPh and NCNR′2, while alkylnitrile
N+(O−)Me demonstrates comparable reactivity of the coordinated NCPh and NCNR′2, while alkylnitrile 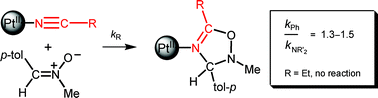

 Please wait while we load your content...
Please wait while we load your content...