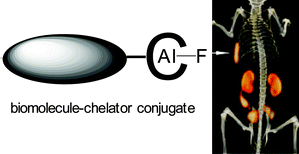
Conventional methods for radiolabelling biomolecules such as proteins and peptides with fluorine-18 for PET imaging rely on carbon–fluorine bond formation and are complex and inefficient. Several non-carbon elements form strong bonds (i.e. with high bond enthalpy) with fluorine, but with lower activation energy for their formation compared to carbon–fluorine bonds, whilst preserving a relatively high kinetic stability. In particular, by incorporating boron-, aluminium- and silicon-containing prosthetic groups into biomolecules, promising results have recently been achieved in the radiolabelling with F-18-fluoride under mild aqueous conditions, affording a level of convenience, efficiency and specific activity potentially superior to those offered by conventional C–F bond formation methods. The promise already shown by these early studies heralds a new branch of bioconjugate radiochemistry involving a wider range of “fluoridephilic” elements for synthesis of PET molecular imaging agents.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...