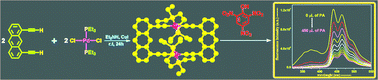
Design and synthesis of three novel [2 + 2] self-assembled molecular rectangles 1–3via coordination driven self-assembly of predesigned Pd(II) ligands is reported. 1,8-Diethynylanthracene was assembled with trans-Pd(PEt3)2Cl2 in the presence of CuCl catalyst to yield a neutral rectangle 1via Pd–C bond formation. Complex 1 represents the first example of a neutral molecular rectangle obtained via C–Pd coordination driven self-assembly. A new Pd2II organometallic building block with 180° bite-angle 1,4-bis[trans-(ethynyl)Pd(PEt3)2(NO3)]benzene (M2) containing ethynyl functionality was synthesized in reasonable yield by employing Sonagashira coupling reaction. Self-assembly of M2 with two organic clip-type donors (L2–L3) afforded [2 + 2] self-assembled molecular rectangles 2 and 3, respectively [L2 = 1,8-bis(4-pyridylethynyl)anthracene; L3 = 1,3-bis(3-pyridyl)isophthalamide]. The macrocycles 1–3 were fully characterized by multinuclear NMR and ESI-MS spectroscopic techniques, and in case of 1 the structure was unambiguously determined by single crystal X-ray diffraction analysis. Incorporation of Pd-ethynyl bonds helped to make the assemblies π-electron rich and fluorescent in nature. Complexes 1–2 showed quenching of fluorescence intensity in solution in presence of nitroaromatics, which are the chemical signatures of many commercially available explosives.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...